Nuclear fission is the process whereby a nucleus is split into two smaller nuclei. Fission happens when a nucleus absorbs an extra neutron and becomes highly unstable, instantly splitting apart. The starting nucleus is known as the ‘parent’ and the split fragments are known as ‘daughter’ nuclei.
The parent nucleus does not split exactly in half but instead produces one daughter that is more than half of the parent’s mass and another that is less than half of its mass.
One of the ways in which fissile uranium (U-235) can split is by producing krypton (Kr) and barium (Ba) as shown in the diagram below.
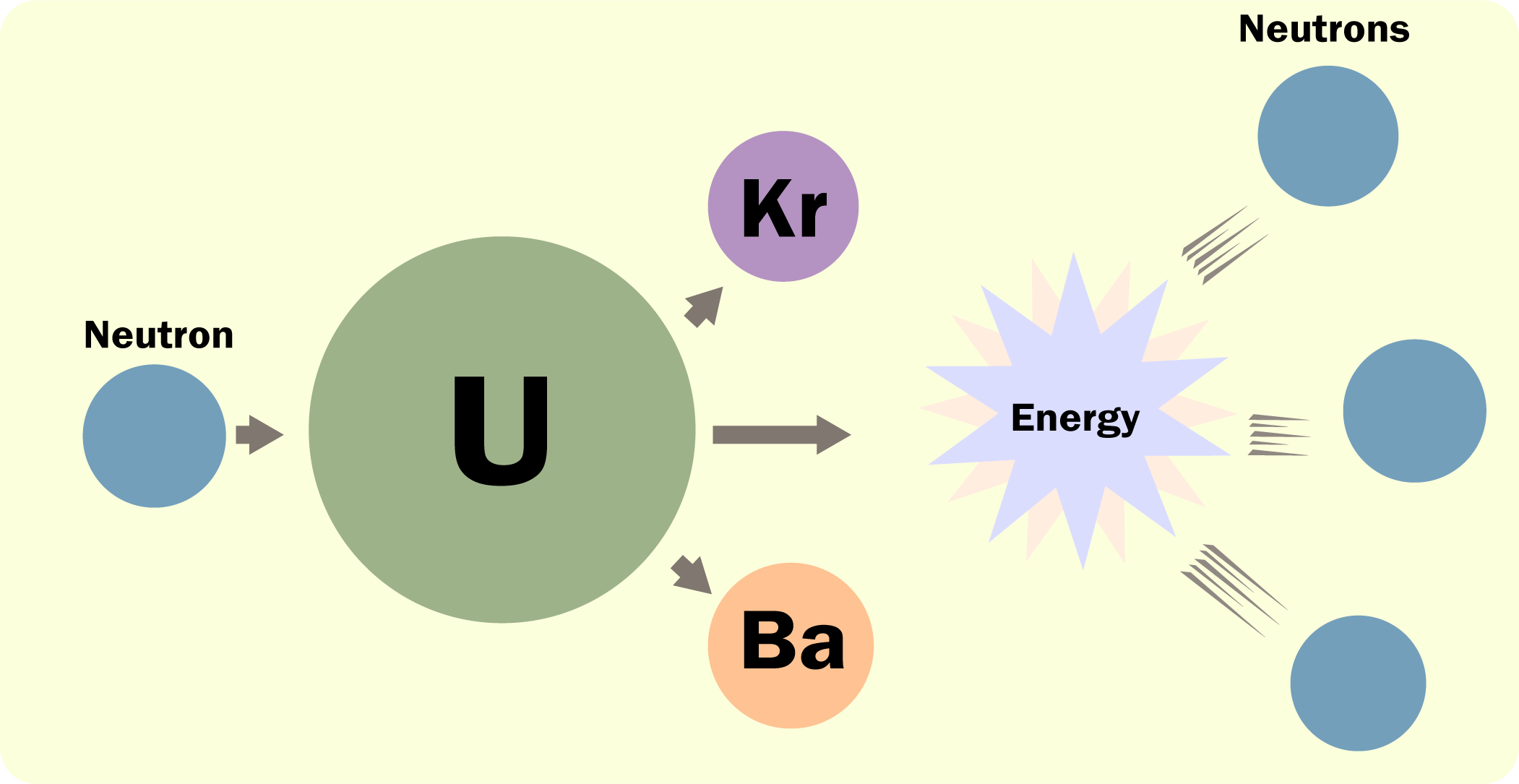
The image above is from the US Environmental Protection Agency’s RadTown educational resources, accessible here (or click here for the original image itself).
You can tell that the parent nucleus has not split exactly in half because the two daughter nuclei are different and neutrons have been released. The atomic number of uranium is 92 whereas the daughter nuclei have atomic numbers of 36 (krypton) and 56 (barium) so it is very obvious that the fissile uranium did not (even approximately) split in half.
To be more accurate about the fission process, we need to look at the atomic mass of the parent and daughter nuclei – but this is more complicated because there are several different isotopes (variants of the daughter nuclei) that can be produced.
In one type of fission, U-235 absorbs one neutron then splits to produce Kr-94 and Ba-139 together with three free neutrons. It is also possible for U-235 to absorb a neutron and split into Kr-91 and Ba-143 with two free neutrons. And to complicate matters even further, uranium can also produce a completely different pair of daughter nuclei (such as xenon and strontium). The average number of neutrons produced for all the possible fissions of U-235 is 2.48 but the exam board only expects you to know that each of the possible reactions produces “two or more” free neutrons.
