At first glance the decay of molybdenum-99 to produce technetium-99 is a straightforward beta process. The total number of nucleons is unchanged but the number of protons increases by one with the emission of an electron and an electron-antineutrino. The half-life for this decay is 66 hours.

In the nuclear decay equation above, technetium-99 is identified in an unusual way, as 99mTc: this is because it is produced in an unstable (metastable) state that will subsequently become stable through the emission of a gamma photon. This type of behaviour is not unique to technetium-99m but in most other cases the intermediate state is extremely short-lived, causing the gamma photon to follow particle emission within picoseconds or less, so the decay can be treated as a single process.
This is not true for technetium-99m, which has its own half-life of just over six hours and therefore warrants a separate decay equation, as shown below.

Metastable technetium-99 can be chemically separated from the molybdenum beta emitter to produce a sample that emits only gamma radiation. This is important because it allows technetium-99m to be used in medical imaging with minimal risk of tissue damage caused by beta radiation.
The gamma photon released by a metastable technetium-99 nucleus carries an energy of 140 keV so it can easily pass through human tissues. This characteristic, combined with a useful six-hour decay half-life and a biological half-life (the rate at which it is cleared from the human body) of about a day, makes metastable technetium-99 ideal for use in medical imaging. A source giving more details about the medical uses of 99mTc is accessible on the US National Library of Medicine website at https://www.ncbi.nlm.nih.gov/books/NBK559013/.
Although they go beyond the A-level physics syllabus, three further points are worth noting as they indicate some of the finer details of radioactive decays that are overlooked to make matters a bit simpler when studying radioactivity in school.
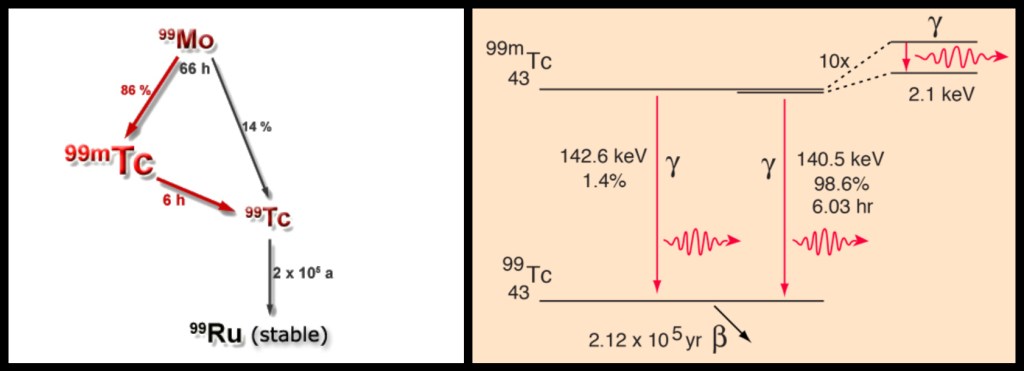
- The final form of technetium-99 is itself radioactive and decays by beta emission with a nuclear half-life of 210,000 years, which is why the short biological half-life mentioned above is important for ensuring patient safety.
- Not every nucleus of molybdenum-99 decays to technetium-99m; a small proportion of the parent nuclei decay directly to the stable form, technetium-99.
- Although a 140 keV photon is by far the most common emission, other energies of gamma photons are also produced by the decay of metastable technetium-99.
The last two of these points are illustrated in the diagrams below.
Further Reading: There is an excellent A-level description of decays from excited nuclear states on the Save My Exams website, at https://www.savemyexams.com/a-level/physics/aqa/17/revision-notes/8-nuclear-physics/8-3-nuclear-instability-and-radius/8-3-3-nuclear-excited-states/

One thought on “Technetium-99m”