Tucked away inside a website of astronomy resources I have just found a brilliant little tool for revising atomic structure, which is required knowledge for AQA Trilogy (and other) GCSE examinations in both physics and chemistry. You can find it at https://astro.unl.edu/newRTs/nuclei/.
The assessment is a two-part, drag-and-drop ranking exercise where you have to rearrange the atoms so that they are in order of increasing number of electrons and (separately) in increasing number of neutrons, as shown in the (non-interactive) screen-grab below.
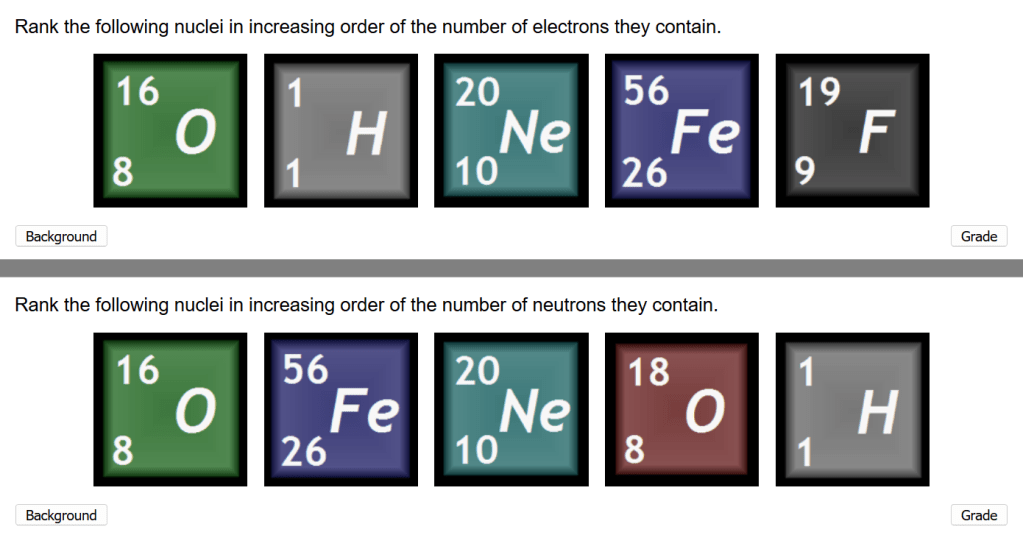
A really nice touch is that sometimes the number of neutrons can be the same for two different atoms. Fortunately, it doesn’t matter which atom you put first as the grading scheme marks both as correct. The bottom row of the example shown above includes this situation, which is made possible by the use of different isotopes.
You should be able to describe what is meant by isotopes, spot the two in the example test above and identify the two atoms that have the same number of neutrons (only one of which is part of the isotope pair).
Refreshing the assessment page will bring up new combinations of atoms so you can have multiple attempts without simply repeating the same question. It’s a great little resource so please do make use of it, at https://astro.unl.edu/newRTs/nuclei/.
If you can’t work out how to get the right answers, which are revealed using the Grade button, then use the following information…
The smaller number next to the atomic symbol is the atomic number. The atomic number is an integer and it is always the same for a specific element. It gives the element’s position in the Periodic Table, counting from the top left corner, going left to right and top to bottom.
The atomic number is equal to the number of protons inside the nucleus and for this reason, it is also known as the proton number: The value of the atomic number is also equal to the number of electrons around the nucleus. Why? Because the charge on a proton and the charge on an electron have equal magnitude but the proton is positive whereas the electron is negative and atoms are always electrically neutral. (Note that the last statement is not true for ions, which are atoms that have either gained or lost one or more electrons.)
The larger number next to the atomic symbol measures the atomic mass. The atomic mass is not an integer, although in simplified Periodic Tables it is often rounded to a whole number.
The atomic mass is equal to the total number of protons and neutrons inside the nucleus. Logically, this means the atomic mass should be a whole number but a single element can take different forms (called isotopes) where the number of neutrons varies. If we average-out the number of neutrons, taking account of the abundance of the different forms, we get a true value for the atomic mass – and that is a non-integer value. (Note that this calculation is a required skill in the chemistry syllabus for Higher tier.)
The number of protons and electrons in an atom is given by the atomic number (as explained above) but to determine the number of neutrons we have to do a minor calculation: deduct the atomic number from the atomic mass to get the number of neutrons.
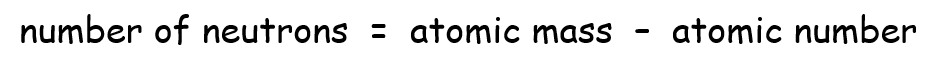
You will only ever have to do this calculation using integer values, as is the case in the assessment recommended here.
